
ResearchResearch
1.E.2 Apo-plastocyanin
The side chain of the cysteine residue of apoplastocyanin (apoPC) was
site-specifically modified with a 4,5-dimethoxy-2-nitrobenzyl derivative,
where the CD and 2D NMR spectra showed that the modified apoPC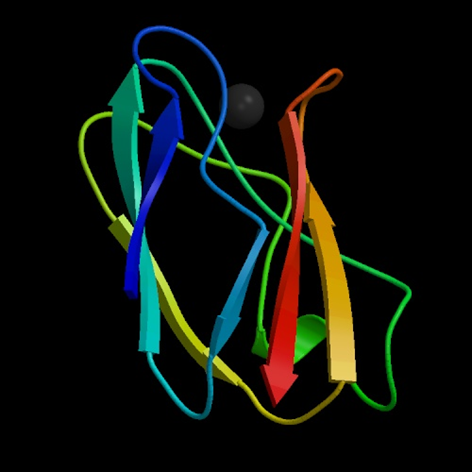 was unfolded. The substituent was cleaved with a rate of about 400 ns
by photo-irradiation, which was monitored by the disappearance of the absorption
band at 355 nm and the increase in the transient grating signal. After
a sufficient time from the photo-cleavage reaction, the CD and NMR spectra
showed that the native β-sheet structure was recovered. Protein folding
dynamics was monitored in the time-domain with the transient grating method
from a viewpoint of the molecular volume change and the diffusion coefficient,
both of which reflect the global structural change including the protein–water
interaction. The observed volume decrease of apoPC with a time scale of
270 μs is ascribed to the initial hydrophobic collapse. The increase in
the diffusion coefficient (23 ms) is considered to indicate a change from
an intermolecular to an intramolecular hydrogen bonding network.
was unfolded. The substituent was cleaved with a rate of about 400 ns
by photo-irradiation, which was monitored by the disappearance of the absorption
band at 355 nm and the increase in the transient grating signal. After
a sufficient time from the photo-cleavage reaction, the CD and NMR spectra
showed that the native β-sheet structure was recovered. Protein folding
dynamics was monitored in the time-domain with the transient grating method
from a viewpoint of the molecular volume change and the diffusion coefficient,
both of which reflect the global structural change including the protein–water
interaction. The observed volume decrease of apoPC with a time scale of
270 μs is ascribed to the initial hydrophobic collapse. The increase in
the diffusion coefficient (23 ms) is considered to indicate a change from
an intermolecular to an intramolecular hydrogen bonding network.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals