
ResearchResearch
1.A.2 Photo-dynamics of octopus rhodopsin
@@Rhodopsins are photoreceptor proteins of many animals. They consists
of 11-cis retinal chromophore covalently attached to the opsin via a protonated
Schiff base linkage. Light isomerizes the 11-cis retinal to its all-trans
form followed by a series of protein conformational changes, called the
photointermediates of bleaching, each usually characterized by their different
absorption spectra. One of the photointermediates interacts with the G
protein, resulting in the electrical excitation of a photoreceptor cell.
Spectroscopic methods have been extensively applied to the study of the
reactions following light excitation. Transient absorption spectroscopy
showed that transformation of mesorhodopsin to acid metarhodopsin is the
final spectral transformation in the photolysis of octopus rhodopsin. However,
it is not certain that these spectrally accessible species are the only
species involved in the reaction. To study kinetics of a process, which
is not accessible by any optical absorption changes, the transient grating
technique or the photoacoustic technique were used to monitor the reaction
volume change.
@@A spectrally silent transformation in the photolysis of octopus rhodopsin
was detected by the time-resolved transient grating method. Our results
showed that at least two photointermediates, which share the same chromophore
absorption spectrum, exist after the final absorption changes. This indicates
that the parts of the protein distant from the chromophore are still changing
even after the changes in microenvironment around the chromophore are over.
From the signal intensity detected by the transient grating method, the
volume change of the spectrally silent transformation was found to be deltaV
= 13 ml/mol. The activation energy of the spectrally silent transformation
is much lower than those of other transformations of octopus rhodopsin.
Since stable acid metarhodopsin has not been shown to activate G protein,
this transient acid metarhodopsin may be responsible for G protein activation.
@@Enthalpy changes (delH) of the photointermediates that appear in the
photolysis of octopus rhodopsin were measured at physiological temperatures
by the laser-induced transient grating method. The enthalpy from the initial
state, rhodopsin, to bathorhodopsin, lumirhodopsin, mesorhodopsin, transient
acid metarhodopsin and acid metarhodopsin were 146 kJ/mol, 122kJ/mol, 38
kJ/mol, 12 kJ/mol and 12 kJ/mol, respectively. It was surprising to know
that the delH of lumirhodopsin at phys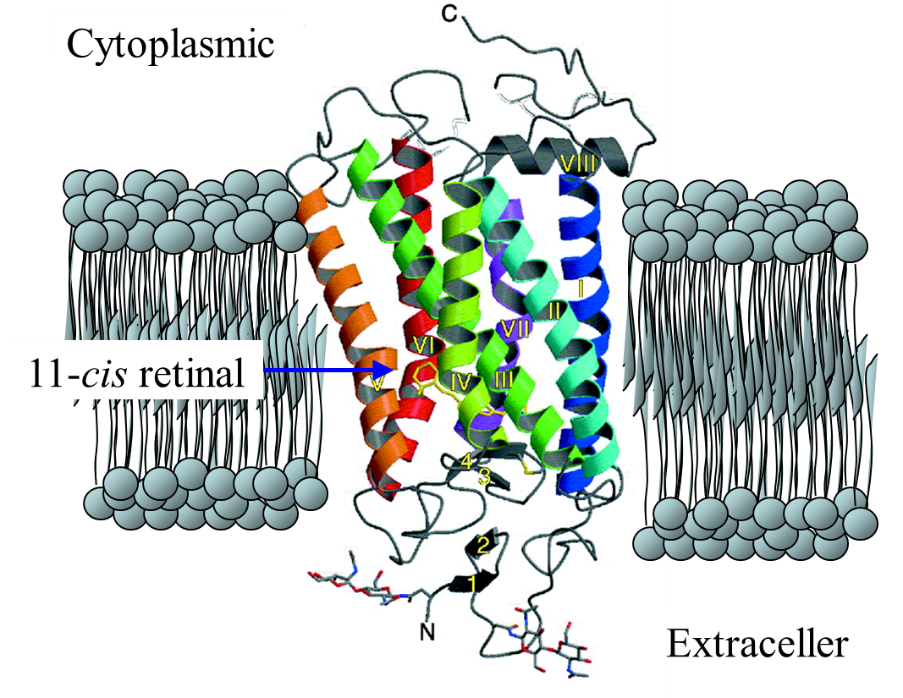 iological temperatures is quite different from that at low temperature.
The reaction volume changes of these processes were determined by the pulsed
laser-induced photoacoustic method along with the above delH values. Initially,
in the transformation between rhodopsin and bathorhodopsin, a large volume
expansion of +32 ml/mol was obtained. The volume changes of the subsequent
reaction steps were rather small. These results are compared with the structural
changes of the chromophore, peptide backbone, and water molecules within
the membrane helixes reported previously.
iological temperatures is quite different from that at low temperature.
The reaction volume changes of these processes were determined by the pulsed
laser-induced photoacoustic method along with the above delH values. Initially,
in the transformation between rhodopsin and bathorhodopsin, a large volume
expansion of +32 ml/mol was obtained. The volume changes of the subsequent
reaction steps were rather small. These results are compared with the structural
changes of the chromophore, peptide backbone, and water molecules within
the membrane helixes reported previously.
@@@@@@@@@@@@@@@@@@@@@@ (Back)


photo-physical-chemistry lab,sεwεw@w€Θ »wκU υ¨»w€Ί
§606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
Links for members
Bake Web mail (Set up)@
Manuals