
ResearchResearch
1.D Photolyase
Light is essential for life, yet ultraviolet (UV) light can be harmful
due to its damaging photochemical reactions with biological molecules.
For example, UV-induced chemical reactions between two adjacent pyrimidine
bases within the same DNA strand produce two major photoproducts: 70-80
% are cyclobutane pyrimidine dimers (CPDs) and 20-30 % are pyrimidine-pyrimidone
(6-4) photoproducts ((6-4) PPs). To maintain genetic integrity, organisms
use self-defense machineries. Photolyases (PHR) are unique DNA repair enzymes,
which light-dependently catalyze the conversion of these photoproducts
to original pyrimidines. Two types of PHRs are known: CPD photolyase (CPD
PHR), which repairs only CPDs, and (6-4) photolyase ((6-4) PHR), which
specifically recognizes and repairs (6-4) PPs. Besides these PHRs, many
higher organisms have PHR-like proteins termed cryptochromes (CRYs), controlling
growth and flowering in plant and daily rhythms in animals. The PHRs and
CRYs have a common choromophore, flavin adenine dinucleotide (FAD). Within
the cryptochrome/photolyase family, the (6-4) PHRs show the highest sequence
and structural similarities to mammalian circadian clock-related CRYs.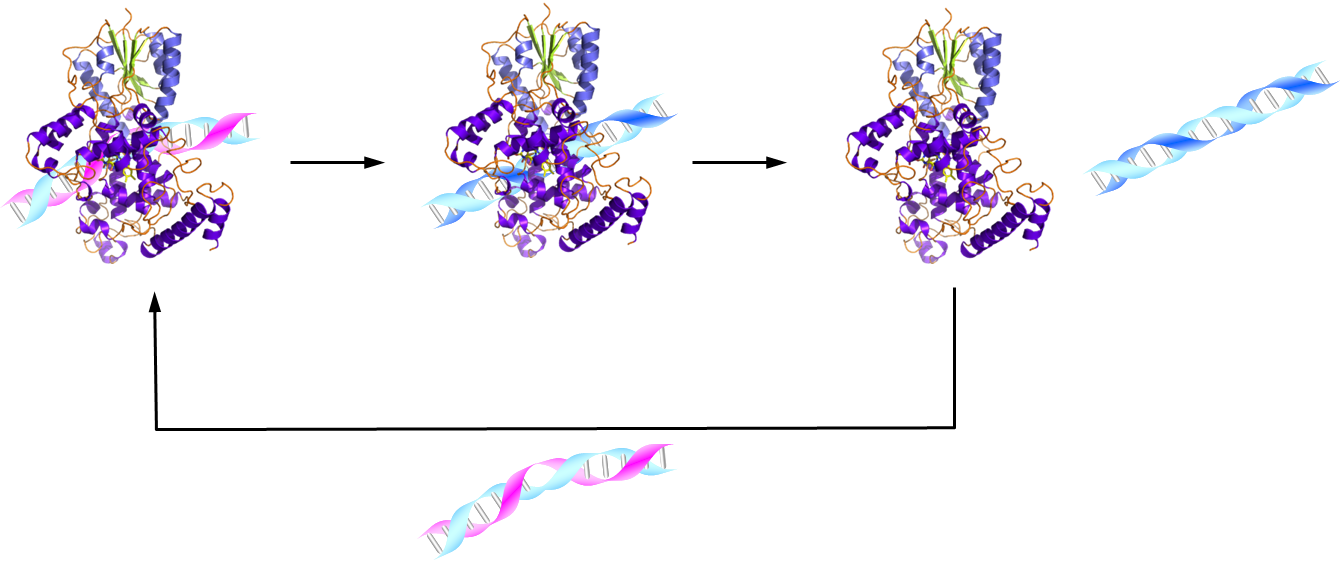
The (6-4) photolyase by itself showed significant volume changes after
blue-light activation, indicating protein conformational changes distant
from the flavin cofactor. A drastic diffusion change was observed only
in the presence of both (6-4) photolyase and damaged DNA, and not for (6-4)
photolyase alone or with undamaged DNA. Thus, we propose that this diffusion
change reflects the rapid (50 s time constant) dissociation of the protein
from the repaired DNA product. Conformational changes with such fast turnover
would likely enable DNA repair photolyases to access the entire genome
in cells.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals