
ResearchResearch
1.A.3 (i) Sensory Rhodopsin from Natronobacterium pharaonis
Dynamics of protein conformational change of Natronobacterium pharaonis
sensory rhodopsin II (NpSRII) and of NpSRII- transducer protein (delNpHtrII)
complex are investigated in solution phase at room temperature by the laser
flash photolysis and the transient grating methods in real time. The diffusion
coefficients of both species indicate that the NpSRII-delNpHtrII complex
exists in the dimeric form in 0.6 % dodecyl--maltopiranoside (DM) solution.
Rate constants of the reaction processes determined from the transient
absorption and grating methods agree quite well. Significant differences
was found in the volume change and the molecular energy between NpSRII
and NpSRII-delNpHtrII complex samples. The enthalpy of the second intermediate
(L) of NpSRII-delNpHtrII is more stabilized compared with that of NpSRII.
This stabilization indicates the influence of the transducer to the NpSRII
structure in the early intermediate species by the complex formation. Relatively
large molecular volume expansion and contraction were observed in the last
two steps for NpSRII. Additional volume expansion and contraction were
induced by the presence of delNpHtrII. This volume change, which should
reflect the conformational change induced by the transducer protein, suggested
that this is the signal transduction process of the NpSRII-delNpHtrII complex.
The interaction between sensory rhodopsin II (SRII) and its transducer
HtrII was also studied using the D75N mutant of SRII, which exhibits minimal
visible light absorption changes during its photocycle, but mediates normal
phototaxis responses. Flash-induced transient absorption spectra of transducer-free
D75N and D75N joined to 120 amino acid residues of the N-terminal part
of the SRII transducer protein HtrII showed only one spectrally distinct
K-like intermediate in their photocycles, but the TG method resolved four
intermediates (K1-K4) distinct in their volumes. D75N bound to HtrII exhibited
one additional slower kinetic species which persists after complete recovery
of the initial state as assessed by absorption changes in the UV-visible
region. The kinetics indicate a conformationally changed form of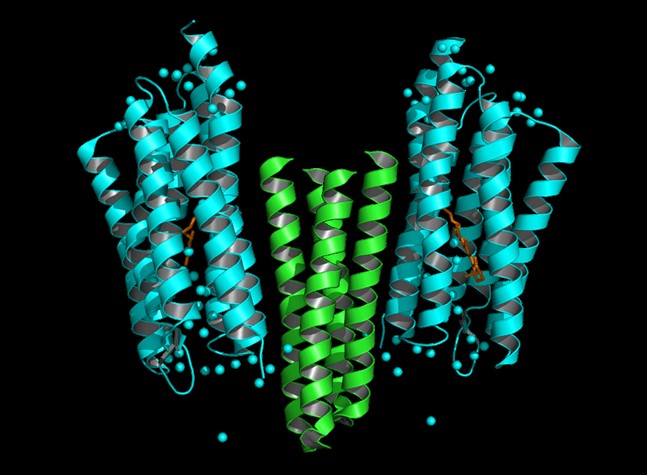 the transducer portion (designated Tr*), which persists after the photoreceptor
returns to the unphotolyzed state. The largest conformational change in
the HtrII portion was found to cause a HtrII-dependent increase in volume
rising in 8 micros in the K4 state and a drastic decrease in the diffusion
coefficient (D) of K4 relatively to those of the unphotolyzed state and
Tr*. The magnitude of the decrease in D indicates a large structural change,
presumably in the solvent-exposed HAMP domain of HtrII, where rearrangement
of interacting molecules in the solvent would substantially change friction
between the protein and the solvent.
the transducer portion (designated Tr*), which persists after the photoreceptor
returns to the unphotolyzed state. The largest conformational change in
the HtrII portion was found to cause a HtrII-dependent increase in volume
rising in 8 micros in the K4 state and a drastic decrease in the diffusion
coefficient (D) of K4 relatively to those of the unphotolyzed state and
Tr*. The magnitude of the decrease in D indicates a large structural change,
presumably in the solvent-exposed HAMP domain of HtrII, where rearrangement
of interacting molecules in the solvent would substantially change friction
between the protein and the solvent.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals