
ResearchResearch
1.A.5 (a) YcgF
The YcgF protein from Escherichia coli is composed of an N-terminal BLUF
domain and a C-terminal EAL domain, which contains an abundance of the
amino acids, glutamine (E), alanine (A), and leucine (L). 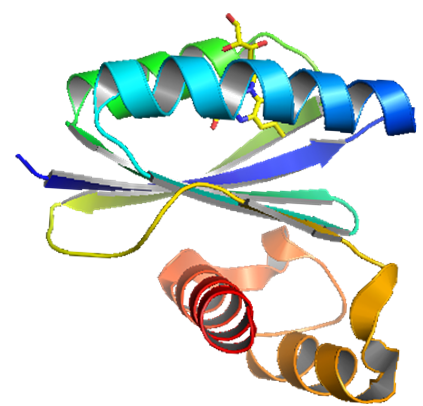 The EAL domain has been shown to encode the enzymatic activity involved
in the hydrolysis of cyclic diguanosine monophosphate (c-di-GMP), which
is a regulatory signaling molecule for various biological events. Therefore,
it has been proposed that YcgF functions as a blue-light regulated phosphodiesterase
(Blrp). The photochemical reaction dynamics of YcgF, a BLUF protein, were
investigated by the pulsed laser-induced transient grating (TG) technique.
The TG signal showed three reaction time constants: 2.7 micros, 13 micros,
and 2 ms. The fastest was tentatively attributed to relaxation of the excited
triplet state of the chromophore, flavin adenine dinucleotide (FAD), and
the others represented conformational changes of the protein. The TG signal
provided clear evidence that the diffusion coefficient (D) of the photo-product
(3.8 10-11 m2s-1) was significantly less than that of the reactant (8.3
10-11 m2s-1), with a time constant of 2 ms at a protein concentration
of 700 microM. Interestingly, the rate constant increased in proportion
to the concentration of the protein, indicating that protein dimerization
was one of the main reactions occurring after photoexcitation. The significant
reduction in D indicates that a conformational change leading to an increase
in interactions with water molecules occurs upon formation of the signaling
state. The 13 microsec dynamics was attributed to the conformational change
(diffusion sensitive conformational change) that induced transient dimerization.
This conformational change might be an essential process for the creation
of the signaling state. A detailed scheme for the photochemical reaction
of YcgF is proposed.
The EAL domain has been shown to encode the enzymatic activity involved
in the hydrolysis of cyclic diguanosine monophosphate (c-di-GMP), which
is a regulatory signaling molecule for various biological events. Therefore,
it has been proposed that YcgF functions as a blue-light regulated phosphodiesterase
(Blrp). The photochemical reaction dynamics of YcgF, a BLUF protein, were
investigated by the pulsed laser-induced transient grating (TG) technique.
The TG signal showed three reaction time constants: 2.7 micros, 13 micros,
and 2 ms. The fastest was tentatively attributed to relaxation of the excited
triplet state of the chromophore, flavin adenine dinucleotide (FAD), and
the others represented conformational changes of the protein. The TG signal
provided clear evidence that the diffusion coefficient (D) of the photo-product
(3.8 10-11 m2s-1) was significantly less than that of the reactant (8.3
10-11 m2s-1), with a time constant of 2 ms at a protein concentration
of 700 microM. Interestingly, the rate constant increased in proportion
to the concentration of the protein, indicating that protein dimerization
was one of the main reactions occurring after photoexcitation. The significant
reduction in D indicates that a conformational change leading to an increase
in interactions with water molecules occurs upon formation of the signaling
state. The 13 microsec dynamics was attributed to the conformational change
(diffusion sensitive conformational change) that induced transient dimerization.
This conformational change might be an essential process for the creation
of the signaling state. A detailed scheme for the photochemical reaction
of YcgF is proposed.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals