
ResearchResearch
1.A.4 (a) phototropin from Arabidopsis thaliana
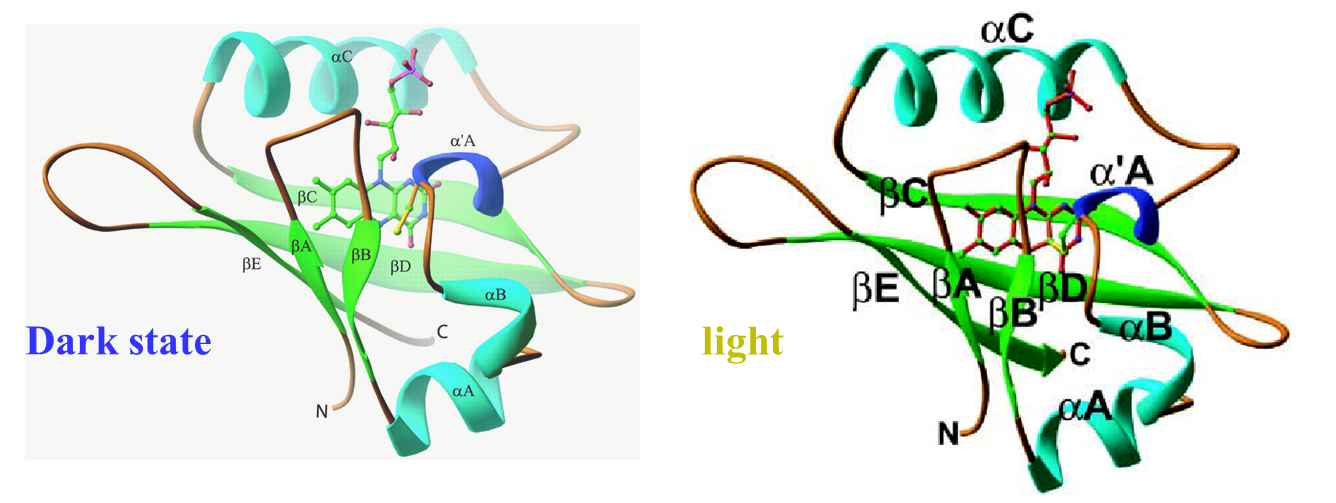
(i) Phot1LOV1
The photochemical reaction of the LOV1 (light-oxygen-voltage 1) domain
of phototropin 1 from Arabidopsis thaliana was investigated by the time-resolved
transient grating method. As with other LOV domains, an absorption spectral
change associated with an adduct formation between its chromophore (flavin
mononucleotide) and a cysteine residue was observed with a time constant
of 1.1 μs. After this reaction, a significant diffusion coefficient (D)
change (D of reactant = 8.2 × 10^−11 m2/s, D of photoproduct = 6.4 × 10^−11
m2/s) was observed with a time constant of 14 ms at the protein concentration
of 270 microM. From the D value of the ground state and the peak position
in size exclusion chromatography, we have confirmed that the phot1LOV1
domain exists as a dimer in the dark. The D-value and the concentration
dependence of the rate indicated that the phot1LOV1 domain associates to
form a tetramer (dimerization of the dimer) upon photoexcitation. We also
found that the chromophore is released from the binding pocket of the LOV
domain when it absorbs two photons within a pulse duration, which occurs
in addition to the normal photocycle reaction. On the basis of these results,
we discuss the molecular mechanism of the light dependent role of the phot1LOV1
domain.
(ii) Phot1LOV2, Phot1LOV2-linker
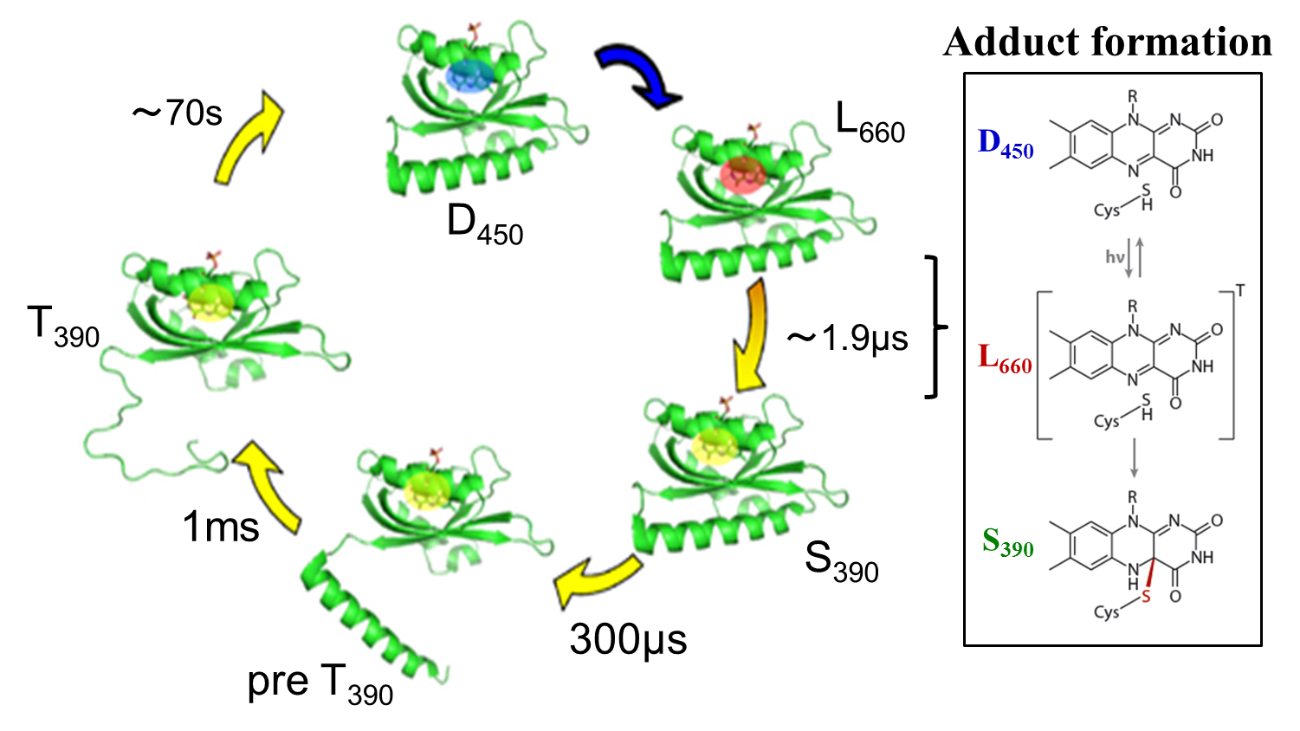
We observed a time dependent D and it was interpreted in terms of the
unfolding of alpha-helices in the linker region. The change of the a-helices
was confirmed by observing the recovery of the circular dichroism intensity.
The TrL signal showed that the molecular volume decreases with two time
constants; 300 micros and 1.0 ms. The former time constant is close to
the previously observed photo-dissociation reaction rate of the phot1LOV2
(without the linker) dimer, and the latter one agrees well with the rate
of the D-change. Considering a similar time constant of the dissociation
reaction of the LOV2 dimer, we interpreted this kinetics in terms of the
dissociation step of the linker region from the LOV2 domain (T390pre state).
After this step, the protein volume and D are decreased significantly with
the lifetime of 1.0 ms. The D-decrease indicates the increase of the intermolecular
interaction between the protein and water molecules. On the basis of these
observations, a two step mechanism of the linker unfolding is proposed.
We also investigated the conformation change of A'a helix in the LOV domain. A mutant (T469I mutant) that renders the A'a helix unfolded in the dark state showed unfolding of the Ja helix with a time constant of 1 ms, which is very similar to the time constant reported for the wild-type LOV2-linker sample. Furthermore, a mutant (I608E mutant) that renders the Ja helix unfolded in the dark state exhibited an unfolding process of the A'a helix with a time constant of 12 ms. On the basis of these experimental results, it is suggested that the unfolding reactions of these helices occurs independently.
(iii) Phot2LOV1, Phot2LOV1-hinge
The TG signal of Phot2LOV1 showed a significant diffusion coefficient
(D) change upon photoexcitation. This change was sensitive to the protein
concentration and the observation time range. These observations were explained
by assuming that there are reactive and non-reactive forms, and the fraction
of these species is concentration dependent. From the concentration dependence
of the dynamics, the monomer was found to form a dimer; however, the dimer
does not exhibit an observable reaction. In the dark state, both species
were in equilibrium and are not distinguishable spectroscopically. For
the LOV1 domain with the hinge domain, the reaction scheme was the same
as the LOV1 domain sample, but the D change was affected by the presence
of the hinge region. This observation suggests that the hinge region undergoes
a conformational change during the photoreaction.
(iv) Phot2LOV2, Phot2LOV2-linker
The diffusion coefficients of the adduct product after forming the chemical
bond between the chromophore and Cys residue of Phot2LOV2 domain is found
to be slightly smaller than that of the reactant, which fact implies that
the core shrinks slightly on the adduct formation. After that change, no
significant conformational change was observed. On the other hand, the
signal of LOV2 with the linker part to the kinase domain clearly shows
very different diffusion coefficients between the original and the adduct
species. The large difference indicates significant global conformational
change of the protein moiety upon the adduct formation. More interestingly,
the diffusion coefficient is found to be time dependent in the observation
time range. This dynamics representing the global conformational change
is a clear indication of a spectral silent intermediate between the excited
triplet state and the signaling product. From the temporal profile analysis
of the signal, the rate of the conformational change is determined to be
2 ms.
(v) Phot2LOV2-kinase
(vi) Phot2LOV1-LOV2
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals